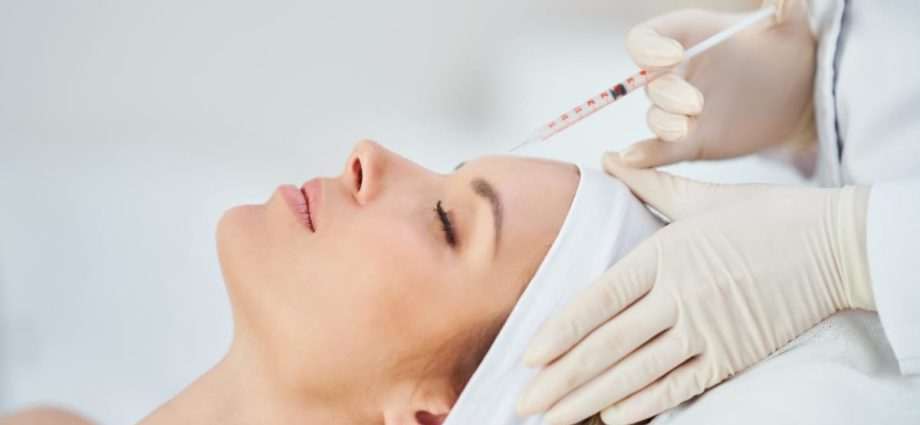
SINGAPORE: According to the company’s distributor, a woman who received dermal fillers during aesthetic treatment went blind because of the procedure, no the actual product.
The dermal filler distributor Parvus -& nbsp, AestheFill, responded to CNA’s questions by saying that its studies revealed that the event” was caused by a blood vessel blockage during the process, which in turn was cause by an unintentional management of the solution into the brain.”
AestheFill is secure and still has Singapore’s Health Sciences Authority( HSA ) approval, according to Parvus.
AestheFill, a model of dermal filler used to temporarily improve facial lines and folds” by shot into the dermal layer of facial skin,” was given to the person in July, according to HSA on September 12.
The case, which Parvus reported on July 29, is being looked into by HSA and the Ministry of Health( MOH ).
According to a representative for Parvus,” We are constantly working with the HSA in their continuing research, and we maintain that item quality and patient security are our top priorities.”
The batch in question has” undergone a comprehensive evaluation, adhering to Korean Food & amp, Drug Administration ( KFDA ) manufacturing standards and complying with the ISO 13485 quality management system ,” the spokesperson continued.
Since October 1, 2021, AestheFill has been registered in Singapore.
According to HSA, this is the first regional adverse event record for dermal filler-related blindness.
Dermal fillers are categorized as Class D medical equipment, which is the highest risk category, in Singapore.
This is a risk inherent in all dermal filler solutions, as shared by several qualified medical cosmetic therapists and physicians since the incident, Parvus said, adding that it should not be injected into blood vessels because it could result in blood vessel blockage and other serious side effects, such as loss of vision.
” Physicians have also shared that problems from visual treatments were largely due to the management technique and not the product ,” according to Parvus.
AestheFill” meets all demanding global safety rules and remains approved by the HSA in Singapore ,” the spokesperson continued. The Singapore Medical Device Register ( SMDR ) still lists the product’s license, which is still in effect today.