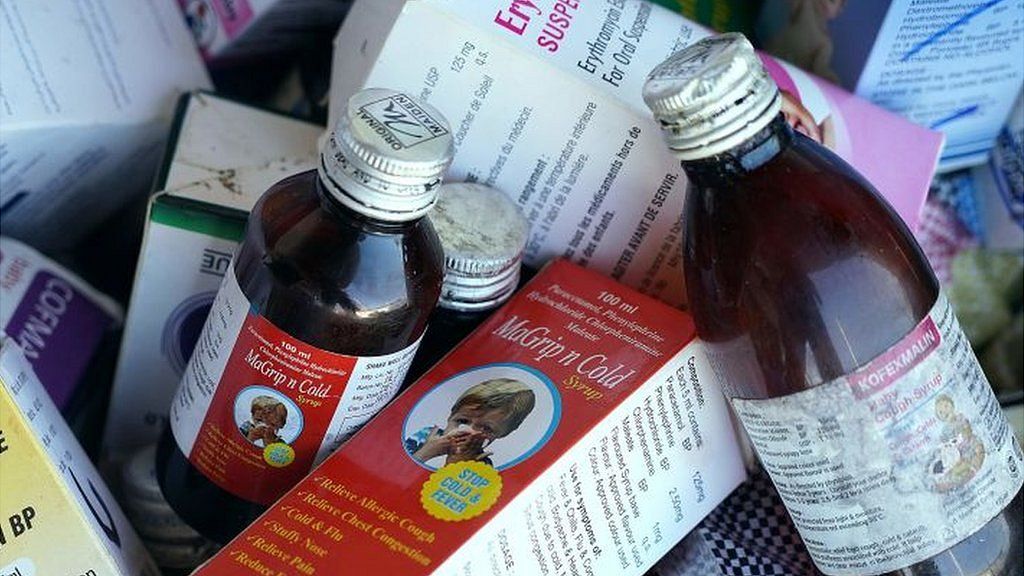
When 17-month-old Nadira fell sick with a cough and flu, her mother Agustina Maulani bought her some paracetamol cough syrup from a health centre in south Jakarta.
“I gave her medicine every four hours, because her fever refused to go down. She would recover, but then have a fever again. In the end she stopped urinating,” she told the BBC.
Nadira was taken to hospital but didn’t get any better. Laboratory diagnosis showed she had excessive levels of urea and creatine; waste products that build up after kidneys shut down. She fell into a coma and died.
“At the end she passed away quickly. With pain that was really terrifying,” said Agustina, crying.
Indonesia is grappling with a horrifying wave of deaths: at least 157 children have died this year from acute kidney injury and other complications, believed to have been caused by contaminated medicines. Almost all were under the age of five.
Nadira died in August. In October the Indonesian authorities said she had been among a wave of children to die of unexplained kidney conditions.
The government then banned the sale of all liquid medicines. It later limited the ban to about 100 suspect products – which had been found in the homes of the children who fell sick.
Pharmacies across the country have pulled bottles from their shelves, advising parents instead to crush pills for their children if they need medicine.
Indonesia’s health minister Budi Gunadi Sadikin said traces of the harmful substances ethylene glycol, diethylene glycol and ethylene glycol butyl ether had been found in the victims.
Diethylene glycol and ethylene glycol are typically used in antifreeze solutions for air-conditioners, fridges and freezers and as a solvent for many products including cosmetics at very low concentrations. The World Health Organization (WHO) says they are never used in medicines.
“It is confirmed that (acute kidney injury) was caused by (those) substances,” the minister said.
The cases come weeks after the deaths of nearly 70 children in The Gambia. The WHO said it found an “unacceptable amount” of diethylene glycol and ethylene glycol in four Indian-made cough syrups available in The Gambia.
There is no suggestion that the two scandals are linked. Indian authorities and manufacturer Maiden Pharmaceuticals say the four syrups were exported to The Gambia only. Indonesia says the Indian-made syrups aren’t available locally.
Last Monday Indonesia’s food and drug agency – BPOM – said it would investigate two pharmaceutical companies that had recently changed their suppliers for some of the ingredients from pharma suppliers to chemical suppliers.
“There are indications in their products… that are highly excessive, highly toxic, and suspected to cause the kidney injury,” BPOM chief Penny Lukito told a news conference.

Meanwhile dozens of sick children are being treated for acute kidney injury and Indonesia has asked Singapore and Australia for supplies of a rare antidote – fomepizole – to treat them.
The tragedy has stunned Indonesia. Last week the country’s Ombudsman criticised the health ministry and BPOM, which it said had not done enough to test products on sale to ensure they complied with standards.
In an enraged editorial, the Jakarta Post newspaper said the BPOM had handed over responsibility for testing to the pharmaceutical companies themselves – a “terrifying” finding that showed the state had “given up its authority”.
“As our hearts are wrenched as parents continue to lose the lives of their precious children, we now find gross negligence and a lack of supervision by the government,” the newspaper said.
Prof Eric Chan at the National University of Singapore told the BBC he was astonished to hear that deaths of this type were continuing to occur – and described events in Indonesia as a “human calamity”.
Diethylene glycol had been used in the past to make medicines taste sweeter – but was now known to be a toxicant, he said.
When diethylene glycol metabolises in the body it forms diglycolic acid that accumulates in and damages kidney cells – it can be life-threatening if not treated in time, he said. A reduction in urination is an early hallmark of kidney poisoning.
Prof Chan said the fact that cases had been seen across Indonesia suggested a pharmaceutical company with wide distribution networks was involved. Medical staff in local hospitals may not be used to handling drug-induced poisoning, he added, borne out by the fact that many of the children were taken from hospital to hospital for treatment.
“We have to keep in mind the number of deaths may still go up,” he warned.

In Bekasi, east of Jakarta, Siti Suhardiyati has forced herself to clear her son’s toys from the floor.
Umar Abu Bakar was two years old when he died on 24 September. He was diagnosed with kidney failure too, by the doctors at his final hospital in Jakarta.
Just two weeks prior he had come down with a fever and a cold. He also had diarrhoea, so Siti took him to a local clinic for treatment.
The family were given three types of medicine, including paracetamol syrup. Three days later Umar stopped urinating.
“I usually change his diapers in the morning as they are full, but this time there was nothing,” she said.
He was taken to a local hospital for treatment before being rushed to Cipto Mangunjusomo Hospital in the capital. But it was too late.
“How can there be stuff that is dangerous in this cough syrup? If it really has been BPOM-approved, it should have been tested,” Ms Suhardiyati said.
Nadira’s mother Ms Maulani is also seeking answers.
“If this was negligence… we demand accountability for the cases that have befallen our children,” she said.