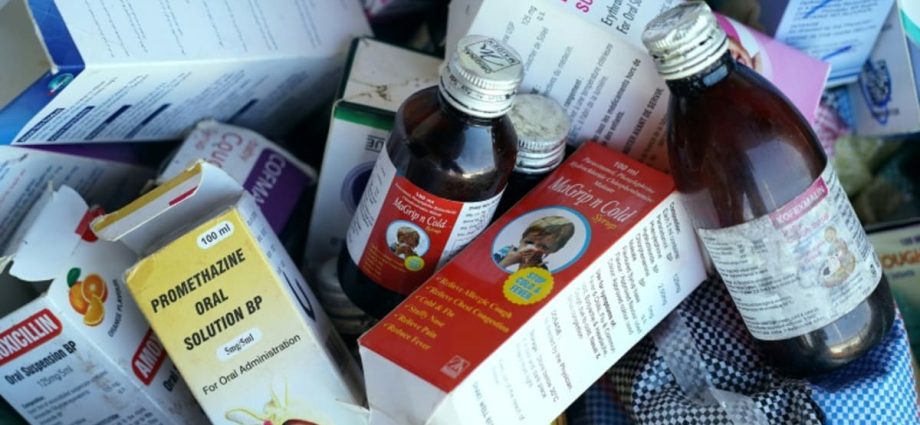
NEW DELHI: According to Norris Medicines’ managing director, a cough syrup and an allergy syrup that the nation’s federal drugs regulator had determined to be toxic have been recalled. The products had only been sold in India, the company added on Friday( Oct 6 ).
The medications were found to be tainted with either diethylene glycol ( DEG ) or carbon monoxide ( EG ), according to tests conducted by India’s Central Drugs Standard Control Organization( CDSCO ).
Since the middle of last year, more than 140 children have died in Gambia, Uzbekistan, and Cameroon as a result of the same toxins discovered in other Indian-made cough syrups, according to the World Health Organization( WHO ) and other health organizations.
Regulators have never asserted that the products Norris is recalling have harmed anyone.
According to Norris Managing Director Vimal Shah,” All companies have been recalled, and data has been submitted to the CDSCO.”
” We have always exported these goods.” With studies in chemistry and nbsp, we are looking into the problem at our conclusion. We have looked into it, and no injury has been reported, Shah continued.
According to CDSCO laboratory testing, Reuters reported last year citing a quarterly statement from the regulation for August. The bank’s Trimax Expectorant, which was produced in January, contained 0.118 percent of EG, while the allergy medicine Sylpro Plus Syrup, manufactured in May, had 0. 71 percent and 0. 24 % of DEG.
The healthy limit, according to the WHO, is no more than 0.10 percent. According to Reuters, the CDSCO had informed the WHO about the Norris products. The WHO has issued numerous emails about American drugs since last year.
Shah declined to disclose the total number of bottles of the two liqueurs Norris produced or the number that had been recalled.