BBC News
BBC World Service
 BBC
BBCIn response to a BBC investigation that revealed two highly addictive drugs were a source of public health problems in some parts of West Africa, Indian officials have banned them.
Dr. Rajeev Singh Raghuvanshi claimed that the authorization to produce and export the medicines had been withdrawn in a text seen by the BBC from India’s Drugs Controller General.
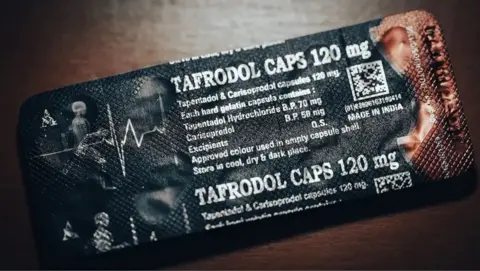
BBC Eye found one pharmaceutical company, Aveo, had been illegally exporting a harmful mix of tapentadol and carisoprodol in countries like Ghana, Nigeria, and Cote D’Ivoire.
The Mumbai factory owned by the company has since been raided, according to India’s Food and Drug Administration, and its whole stock has been seized.
The round from Dr Raghuvanshi, dated to Friday, cited the BBC research in his decision to ban all mixtures of fact and effects, which was to be implemented with quick effect.
He added that this was done after authorities “examined the potential for drug abuse and its detrimental effects on inhabitants”
Carisoprodol is a muscle relaxant, and prompts is a potent opiate, making it so addictive that it is illegal in Europe.
In the US, four is permitted for short-term use up to three weeks. Withdrawal indicators include stress, depression and hallucinations.
The combination of the two medicines, which have breathing problems and convulsions, and which have fatalities when overdosed, is not permitted to be used anywhere in the world.
Despite the risks, these drugs are common street medicines in many East African countries, because they are so affordable and widely available.

Publicly-available trade statistics show that Aveo Pharmaceuticals, along with a girl firm called Westfin International, has shipped thousands of these devices to Ghana and other North American states.
On the pavements of Nigeria and in Ivoirian towns and cities, these medications with the Aveo brand were likewise discovered for sale by the BBC World Service.
Nigeria, with a population of 225 million individuals, provides the biggest market for these medications. According to the government’s National Bureau of Statistics, it is estimated that about four million Nigerians use some form of narcotic.
In one of Aveo’s factories in India, where they filmed one of its executives Vinod Sharma, showing off the same hazardous materials the BBC found for sale in West Africa, the BBC also sent an incognito agent posing as an American entrepreneur looking to sell opioids to Nigeria.
The executive claims in the quietly captured video that Sharma’s goal is to sell the pills to Nigerian teenagers,” who all adore this product.”
Sharma replies,” OK,” before explaining that if people take two or three supplements at once, they may “relax” and accept that they can get “high.”
Towards the end of the meeting, Sharma says:” This is very dangerous for the health”, adding that “nowadays, this is company”.
When the BBC’s preliminary research was released, Sharma and Aveo Pharmaceuticals did not respond to a request for comment.
In a statement released on Friday, India’s Food and Drug Administration said a bruise procedure resulted in the seized of Aveo’s whole property and the suspension of farther production. More legal action will be taken against the business, it added.
The organization stated that it was “fully ready” to take legal action against anyone who engages in “illegal activities that tarnish the country’s popularity.”
Additional inspections have been ordered by the FDA to stop the drug source, according to the statement.


